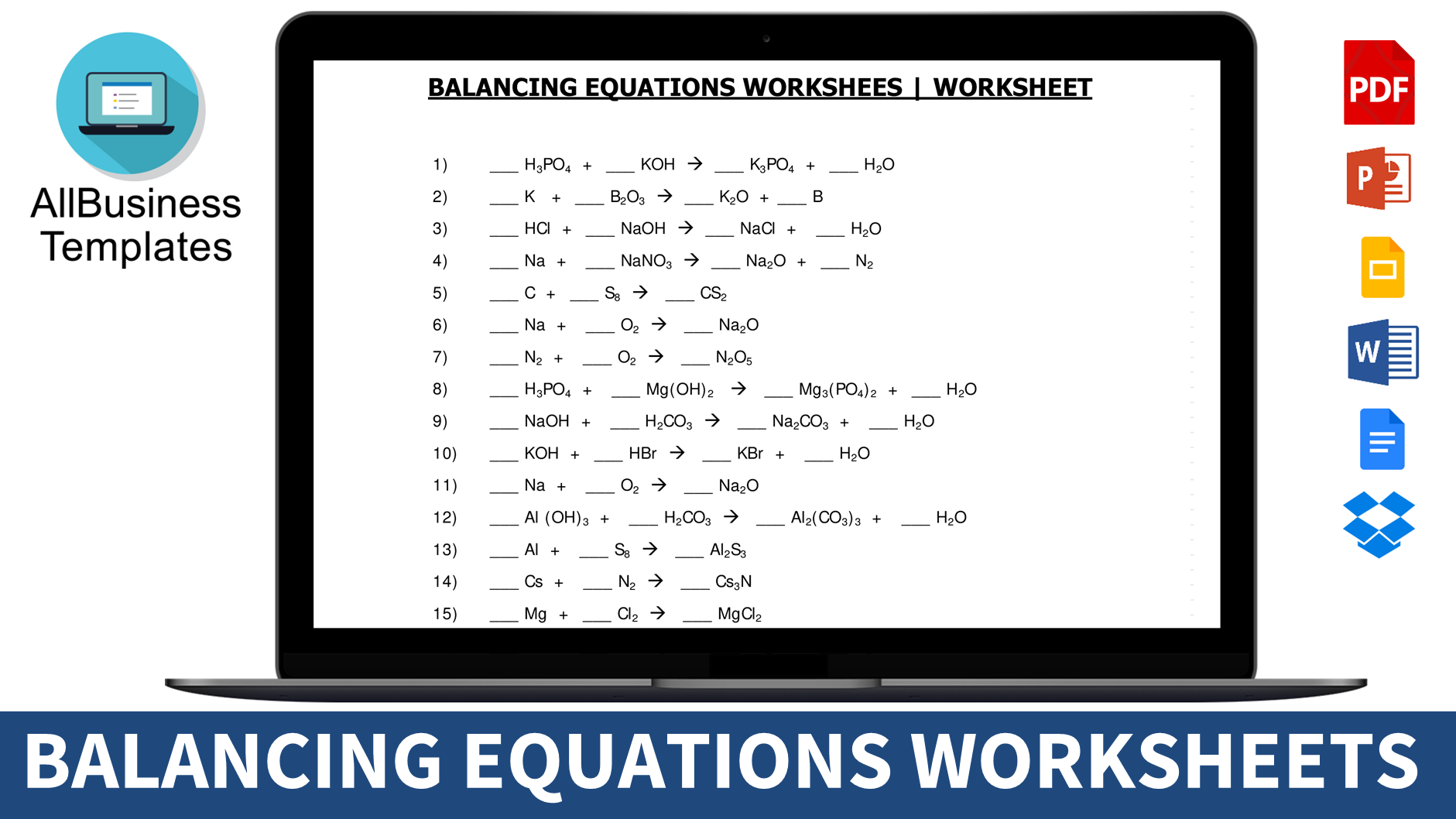
Balancing Equations Worksheet
Speichern, ausfüllen, drucken, fertig!
Are you grappling with the intricacies of balancing chemical equations? Download this free Balancing Equations Worksheet here.
Verfügbare Gratis-Dateiformate:
.pdf- Dieses Dokument wurde von einem Professional zertifiziert
- 100% anpassbar
Education Bildung math Mathematik Science Wissenschaft Chemistry Chemie Printable Worksheets Druckbare Arbeitsblätter
Are you grappling with the intricacies of balancing chemical equations?
The dance of molecules and atoms may leave you bewildered, but fear not! Balancing equations is a must, in accordance with the Law of Conservation of Matter. However, mastering this skill involves practice, knowledge of reactions, formulas, valences, symbols, and techniques, which can often leave students feeling overwhelmed. If you find yourself struggling, look no further – our balancing equations worksheet with answers is here to guide you.
Unlocking the Secrets of Balancing Chemical Equations:
Understanding the methods and tips can significantly ease the process of balancing chemical equations. Achieving balance in an equation establishes a mathematical relationship between products and reactants. If you frequently face challenges in this endeavor, explore the ins and outs and valuable tips for balancing chemical equations in the article below.
Demystifying the Chemical Equation:
A chemical equation serves as the symbolic language of chemistry, portraying chemical reactions through chemical formulas. It encapsulates the reactants and products, showcasing the elements involved in the reaction. With reactants on the left and products on the right, separated by an arrow, chemical equations lay the groundwork for understanding the transformations occurring during reactions.
The Crucial Role of Balancing:
Why is it imperative to balance chemical equations? The Law of Conservation of Mass dictates that the number of atoms on both sides of the equation must be equal. This law, established by Antoine Laurent in 1789, asserts that matter cannot be created nor destroyed. Unbalanced equations, despite correct elements and quantities, are deemed inaccurate and cannot be utilized in calculating chemical reactions.
Diverse Types of Chemical Equations:
Before delving into the tips and tricks of balancing equations, familiarize yourself with the five primary types of chemical reactions:
- Combination or Synthesis Chemical Reaction
- Decomposition Chemical Reaction
- Displacement or Replacement Reaction
- Combustion Reaction
- Acid-Base Reaction
Practical Balancing Equations Worksheet:
For those seeking hands-on practice, our balancing equations worksheet provides an opportunity to refine your skills. Download the worksheet and use it to cross-check your chemical equations. As you prepare for exams, these worksheets serve as valuable tools for honing your proficiency. Best of luck!
How to Balance a Chemical Equation:
If the task of balancing chemical equations appears daunting, follow these steps:
- Write Down the Unbalanced Equation: Begin by listing the chemical formulas of reactants and products. This unbalanced equation forms the foundation for the balancing process.
- Balance the Equation: Apply the Law of Conservation of Mass by ensuring an equal number of atoms on both sides. Start by balancing elements with only one reactant and product, then proceed systematically until all elements are balanced.
- Indicate the States of Matter: Specify the states of matter for products and reactants using symbols such as g for gas, l for liquids, s for solids, and aq for substances in a water solution.
Methods for Balancing Chemical Equations:
Choose between the combustion reaction method and the proportion method for balancing equations, depending on the specific type of chemical equation.
Tips for Balancing Chemical Equations:
- Adjust coefficients, not subscripts.
- Balance polyatomic ions as a whole.
- Start by balancing elements with the highest quantity.
- Verify that the number of atoms is equal on both sides.
- Check coefficients for the lowest terms.
Limitations of Chemical Equations:
- May not clarify the state of substances.
- Does not provide information about reaction speed.
- May lack concentration details.
- Does not indicate color changes or discoloration.
- Does not convey information about reaction speed.
- Some reactions have diverse effects.
Conclusion:
For students grappling with balancing chemical equations, our website offers a comprehensive balancing equations worksheet with answers.
Download it to practice and enhance your skills. With dedication, patience, and practice, you'll conquer the challenges of balancing equations. Keep striving, and success will surely follow!
HAFTUNGSAUSSCHLUSS
Nichts auf dieser Website gilt als Rechtsberatung und kein Mandatsverhältnis wird hergestellt.
Wenn Sie Fragen oder Anmerkungen haben, können Sie sie gerne unten veröffentlichen.

