HTML Preview Analytical Test Method page number 1.
Analytical Test Method Validation Report Template
1. Purpose
The purpose of this Validation Summary Report is to summarize the finding of the validation of test method
“Determination of ……”, following Validation Protocol “….”.
2. Scope/Test Method Description
Optional: ma
y be restated from the protocol.
3. Background
Optional: may be restated from the protocol.
4. Strategy
Optional: may be restated from the protocol.
5. Procedures
Include synopsis of procedure for execution of validation of each characteristic. Include acceptance criteria for
each characteristic.
5.1. Accuracy
Accuracy should be reported as percent recovery of the known added amount or as the difference
between the mean and the accepted true value together with the confidence intervals.
The concentration ranges used should be stated, as should the number of replicates tested.
In the table, the terms concentration 1, 2, and 3 should be replaced with the actual concentrations used
in the experiments. Additional rows may be added if more concentrations were tested.
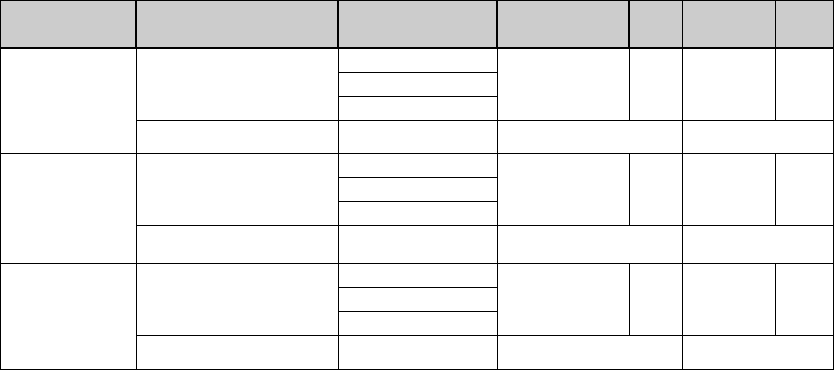
Table: % Recovery & Difference between Mean and Accepted Value
Analyte
Level
Actual
Concentration
Individual %
Recovery
Mean %
Recovery
P/F % RSD P/F
Level 1
Acceptance Criteria
Level 2
Acceptance Criteria
Level 3
Acceptance Criteria
5.2. Precision
5.2.1. Method Precision
Describe the experiments to determine repeatability. Appropriate table shells for this section are
provided below. This table assumes 3 replicate sample preparations at 3 concentrations and assumes
Page1of6